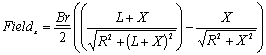[This is an updated repost of an earlier fact-checking article of Breaking Bad.]
It’s likely that people remember more chemistry from an episode of AMC’s Breaking Bad than from their time in high school and college, but is the on-screen science sound? For those who aren't caught up with the exploits of Walter White (Bryan Cranston), the show tracked his transformation from chemistry genius to high school science teacher to cancer patient to meth kingpin. Not only does Walt's scientific background aid him in his drug-making ventures, it also gets him out of scrapes. Where other crime lords in the making might simply fight or shoot their way out of trouble, Walt uses not just his understanding of chemistry, but also physics, toxicology, and biochemistry to dispose of bodies, erase damning evidence, and to make sure that leverage is always on his side. But does TV studio science check out in real life?
While the results on TV might not match up with their real-life counterparts, Breaking Bad sure has done its part for bringing a little bit of scientific curiosity into our living rooms, along with the most compelling drama in recent years. The show was about as perfect as you could get, pseudo-science aside, so I'll happily suspend my disbelief and enjoy the transformation of Walter White from meek to mad scientist. If a little bit of scientific curiosity was sparked along the way, all the better for it!
*Here's our disclaimer: This article is not intended to instruct readers in the methods of explosive production, body disposal, illegal drug manufacturing or to aide in the obtaining of deadly poisons or other unlawful materials. Rather, the following is meant only to compare the Breaking Bad version of science to real world fundamentals. Collider has long been a
money-laundering operationreputable business in good standing. Please contact our attorney, Saul Goodman, with any legal concerns.*
Fulminated Mercury
Two of Breaking Bad’s most famous words (other than the title itself, and the name of protagonist Walter White), “fulminated mercury” resulted in quite the explosive stand-off between Heisenberg and drug kingpin, Tuco Salamanca (Raymond Cruz). Walt cooks up the compound and passes it off as crystal meth, using a small amount to demonstrate its potent properties while holding Tuco’s goons at bay with an amount that would likely level the building. Would this have worked in real life?
Luckily, the folks over at Discovery's Mythbusters have already tackled this one, so I won't have to fire up the ol' fume hood. Fulminated mercury [Hg(CNO)2] is indeed an explosive, classically used as a trigger in blasting caps used to set off larger explosives. The crystal structure of mercury fulminate was discovered in 2007, over 200 years after the compound was first synthesized and about a year before it gained popular attention on the Breaking Bad season one episode, "Crazy Handful of Nothin'". It's decidedly unstable and can be detonated by friction, heat, spark, or shock, such as slamming a few ounces of it against the floor. However, the Mythbusters showed that, in order to get the explosive effects shown in the episode, not only would Walt need a much greater quantity of the compound along with a much faster throwing velocity, but that he and everyone else would have died from the concussive blast.
Compare the fulminated mercury seen in Breaking Bad versus a real chemist's result on Mythbusters, and note the dissimilarities in appearance:
Hydrofluoric Acid
Another nasty bit of science at work in the criminal underworld of Breaking Bad was put to the test by the Mythbusters. This particular scene involved Jesse Pinkman (Aaron Paul) attempting to dispose of a body by submerging it in hydrofluoric acid (HF). Even though Walt warned him that this particularly reactive solution could only be stored in plastic, Jesse went ahead and dumped the body in a bathtub along with a couple gallons of HF. A short while later, the body had been reduced to soup and the acid had also eaten through the tub and the floor beneath it, causing quite the cleanup for Jesse.
Here's the Mythbusters test:
Too bad the HF didn't dissolve anything but the drywall, though it did soften up the pork tissue a bit. The issue here is that, even though the fluorine ion is very reactive, a solution of hydrofluoric acid does not fully dissociate (ie split apart into H and F ions) in water. Since the ability of the compound's ions to dissociate is related to the strength of the acid, HF is considered to be relatively weak. That's not to say it isn't dangerous. It can indeed corrode metal given time and will eat away at the silicon oxide bonds in glass. Skin contact with the solution can lead to painless burns, tissue damage, cell metabolism interference, cardiac arrest and eventually, death. Oh, and in gaseous form, it can damage lungs and the corneas of the eyes. Fun!
The Mythbusters upped the potency and the volume by attempting to dissolve an entire pig in six gallons of sulfuric acid (versus two gallons of HF used by Jesse in Breaking Bad). The pig went bye-bye relatively quickly (not to mention a noxious plume of smoke), but the bathtub and floorboard beneath it remained intact. The Breaking Bad guys apparently wanted to keep the in-series facts canon since they used HF to dispose of more bodies over the next few seasons.
Phosphine Gas
Continuing our tour of deadly chemicals, it turns out that methamphetamine isn't just a deadly and addictive drug, the process to make it is chock full of dangerous and toxic chemicals (shocker). While this should be viewed as an additional deterrent for, ya know, not cooking meth, Walt uses his knowledge of the cook to subdue his less-informed co-workers who happen to be holding him at gunpoint. Knowledge is power! But is it accurate?
In the Breaking Bad pilot, Walt is forced to reveal his recipe so a couple of thugs won't kill him and leave him in the desert. As Walt is cooking in the RV lab, he takes advantage of the dangerous nature of the process to fill the enclosed space with a deadly gas. The cooking of meth involves a chemical reduction of pseudoephedrine or ephedrine (a stimulant and decongestant in cold medications that used to be readily available in drug stores) via red phosphorus and hydriodic acid (another nasty acid for the reasons described above). Red phosphorus is produced by heating its allotrope (different structural form of an element), white phosphorus. On its own, white phosphorus can self-ignite and burn down your makeshift DEA-approved commercial meth lab. When mixed with sodium hyrdoxide, the deadly phosphine gas is produced. The colorless and flammable gas wreaks havoc on the respiratory system when inhaled and is immediately life-threatening at 50 ppm (parts per million, or 0.005% of the volume of breatheable air). Good thing Walt and Jesse had those respirators, otherwise they'd have been in bad shape.
Fun with Flames
It's not all explosions and death and MacGyver saves from life-threatening situations. Sometimes Walt just tries to educate people in the ways of science. In the pilot, we see Walt attempting to fire up the curiosity of his high school chemistry students by spraying various solutions across an open flame, whereupon they change color. His students didn't care, but if you're still reading, I'll wager you're interested.
Here's where we jump from the chemistry lab to the physics lab as this display has more to do with excited energy states than chemical interactions. We get to visit the first law of thermodynamics, which explains that energy is conserved, ie it cannot be created or destroyed, but rather transformed. Walt fires up the Bunsen burner which acts as a heat source. When he sprays a series of flammable solutions across them, the flame flares green and then red. The trick is in the mix, as Walt has likely dissolved metal ions/salts into an ethanol solution. While the ethanol quickly burns off, metal ions are left behind. These ions absorb the thermal energy from the flame, causing their electrons to jump up a notch to an excited state, ever so briefly. Since that state is unstable, the electrons return to their more tenable ground state, but where does that thermal energy go? Ah, it is emitted as light! A copper or barium solution produced the green color, while the red was likely due to strontium. See how you can perform a more advanced presentation of the same concept here.
Effects of Ricin
Yeesh, from fun with science to death by ricin. Well, here we go. Sometimes you don't have to set up an elaborate lab to combine various chemicals with heat and pressure to form toxic compounds ... nature already does that for us! (And Wikipedia posts the helpful header, "Not to be confused with Rice" on their Ricin page. That would be unfortunate, indeed!) Ricin is a toxic compound found in the seeds of the castor oil plant, Ricinus communis. A chemical compound known as a lectin, ricin interferes with the body's most basic cellular ability to produce proteins, throwing a wrench in the works of metabolic processes essential to life. When inhaled or injected, ricin can kill on the order of 22 micrograms per kg (about 1/228th of a standard aspirin tablet, which is why the substance being detected on envelopes sent through the mail is such a serious matter), but it takes about 1,000 times more to kill someone when taken orally (still not that much, ie about four aspirin tablets). Humans can also be poisoned by consuming the castor beans themselves (about 5-20), but should recover with treatment. [Fun fact: Ricin is known as a type 2 ribosome-inactivating protein, with the clever acronym of RIP.] The scariest part about ricin is how easy it is to obtain the plants and process the beans.
As of the writing of this article, the ricin remains in the mix and we're all waiting to see just how Walt intends to use it, if at all, in the finale. He already showed some restraint (or just pure maniacal machination) in not using ricin to poison Brock, even though he managed to harm the little guy in another manner. Which brings us to...
Lily of the Valley
More things in nature can kill us; what a surprise! Walt went through the trouble of processing ricin in order to off a number of bad guys, but it hasn't actually been used to kill anyone (yet). It's the Chekhov's gun of Breaking Bad, and it's been used far more in mind games than in successful assassinations. Walt's best use of ricin (so far) has been making Jesse expose his own mistrust for Walt when the ricin went missing and Brock coincidentally turned up sick. When it was revealed that Brock had been poisoned by eating berries from the Lily of the Valley plant, everyone involved thought Walt was in the clear ... until a closing shot showed Walt sitting comfortably poolside near a potted plant of Lily of the Valley. But is the plant really that toxic?
Yep! All parts of this sucker are harmful, not just the berries (although those are particularly attractive to young children due to their red color). To date, about 38 chemical compounds known as cardiac glycosides occur in Lily of the Valley (Convallaria majalis). These compounds can be used therapeutically in the treatment of cardiac failure, as they increase the force of the heart's contraction by indirectly regulating cellular calcium concentrations. However, when the heart is functioning normally to begin with, ingestion of the Lily of the Vally berries can mess with its rhythm, in addition to imparting blurry vision, diarrhea, vomiting and nausea, disorientation, drowsiness, headaches, red skin rashes, excessive salivation and possible death. It's particularly dangerous to young children and pets.
Methylamine and the Dilution Effect
Not all solutions are created equal. In "Dead Freight", the fifth episode of season five of Breaking Bad, Walt explains why the physical property of density will actually assist them in their planned heist of 1,000 gallons of methylamine from a train transport. Why methylamine? Well, it's an alternate precursor chemical for their cook (one that makes it easier to obtain a higher purity of product), and boosting a large amount from a train was apparently less conspicuous than smurfing dozens of pseudoephedrine-containing products from drug stores. While they can take 1,000 gallons of a 40% w/w (weight-to-weight, not Walter White) solution of methylamine in water, they need only replace that amount with a lesser amount of plain ol' water, mass for mass, not volume for volume. Why? Density differences.
Density is defined as the mass of a substance per unit volume, in other words, how much of something you can cram into a certain space. Pure methylamine has a density of 700.00 kg/m³, but a 40% w/w solution in water works out to about 890 kg/m³. Water, at 20 °C, has a density of 998.21 kg/m³. Now for some fun with conversions: 1,000 gallons = 3.79m³. Multiplying that volume of methylamine by its density gives us a mass of 3,373kg that needs to be replaced with water. Since water has a higher density, a volume (mass divided by density) of only 3.38m³, or 892 gallons, is needed to replace the mass lost in the heist.
Viewers were also worried about the dilution factor of replacing 1,000 gallons of methylamine (keep in mind it's already diluted to 40% w/w) with about 900 gallons of water. I'll spare you another round of calculations. Luckily, the stalwart folks over at Weak Interactions have already taken care of this and provided a nice fact check at the same time. (They've also got an ongoing breakdown of screen science seen in Breaking Bad, so definitely check their blog out if this interests you.) According to their number crunching, the resulting loss in mass is only 0.03% with a final solution of 38% methylamine w/w, amounts that would likely be within tolerance ranges for both the industrial scales and ultimate lab measurements, even though the buyers would quickly be having words with the supplier were this reduction in concentration to become a frequent occurrence.
Fun fact: Walt and Jesse could have simply cooked up methylamine by more or less bubbling ammonia through methanol, but that's not nearly as much fun as a train heist. Plus, Walt would have had to send Jesse to the store for supplies ... and we all know how well that worked out:
Yeah, Bitch! Magnets!
In the season five premiere, Walt and Jesse need to destroy video evidence on a laptop. Doesn't sound too complicated until you realize that said laptop is in the highly protected police evidence locker. Any plans of infiltrating the building will likely get them nabbed in the process, so they dynamic duo decide to attack from without. The place is built like a bunker, so bombs are out of the question. What did they decide? I'll let Jesse explain.
Sounds great in theory (and it was certainly done in entertaining fashion on screen), but would it work in real life? While we wait for the Mythbusters to tackle this one in the future, let's take a look at some data collection the folks at Wired and PC Mag did in order to try and answer this question. First of all, let's focus on the theory behind trying to wipe the computer's memory via a magnet. Traditional hard drives are broken down into tiny domains of uniform magnetization (ie, all pointing up or all pointing down). Translate the up or down orientation into 1s and 0s, and it becomes easy to use binary as a method of information storage. A read-write head can move over the disk and change those domains via a small permanent magnet.
So it sounds like a strong magnet would conceivably reset the data to corrupt it, right? There are even devices, known as Degaussers, which have the sole task of bulk erasing hard drive data. Unfortunately, hard drives need quite a strong magnetic field to force erasure of data (and if you've got a non-magnetic solid-state drive, you're out of luck), and laptops have a significant amount of shielding around their hard drives, so magnets aren't foolproof, as evidenced by this image from K&J Magnetics showing two Neodymium magnets failing to scramble a running hard drive. And just because they won't erase hard drives, don't get these things near your credit cards or old VHS/audio cassettes.
So magnetic erasure is likely out of the question, but what about wanton destruction? Walt and Jesse cranked up the power on the electromagnet so high that it completely wrecked the evidence room (leading to unforeseen complications), including the laptop in question. There's no doubt that electromagnets and rare earth magnets possess fantastic strength, but they also require a target with adequate mass and are more effective over small distances. Maybe this equation will help clarify things:
Yeah ... nevermind. Let's just say that the strength of a magnetic field drops roughly exponentially over distance. Because Walt's electromagnetic was probably a good 5 meters (~15 feet) away from the laptop in the evidence locker (not to mention the reinforced wall between the two), it would have needed quite a bit more power than even those dozens of car batteries could have supplied. They would have been better off seeding clouds above the station and rigging a lightning rod to zap the insides of the evidence locker with a bolt from the blue. Here's a look at how the crew pulled off the TV science magic:
The Dangers of Over-Carbonating Your Schraderbräu
While cooking up meth in your home is very, very illegal, American citizens have been lawfully homebrewing beer and wine for centuries (except for one particularly dry time from 1921 to 1933). Homebrewing relatively small batches of the potent potables is nowhere near as dangerous as cooking up illicit drugs with deadly chemicals, but that’s not to say it’s without its own dangers. Anyone who has ever been splashed with boiling wort or cleaned brewing equipment without proper ventilation knows about these hazards. One desirable byproduct of brewing is the carbon dioxide produced during fermentation, which can form a deadly suffocating atmosphere in fermenting vessels at a concentration of 7% to 10%. CO2 is also responsible for providing fizzy carbonation to alcoholic beverages, but as Hank Schrader (Dean Norris) found out, over-carbonation can have some unintended consequences.
While the bulk of carbon dioxide is produced during alcoholic fermentation, homebrewers dose each bottle with a measured amount of priming sugar in order to better control carbonation. The residual yeast in each bottle metabolize the sugar and churn out more waste products, among them ethanol, various flavor compounds, and, of course, carbon dioxide. However, as Hank discovered, over-carbonating your bottled beer can lead to pressure build up inside the bottles. That pressure finds the path of least resistance (ie the bottle cap) to escape, which sounded remarkably like gunshots in the Breaking Bad episode, “Breakage”.
(Fun fact: bottle and can design takes a remarkable amount of engineering to prevent such things from happening. The next time you’re in a beer/wine store, check out some of the larger bottles of beer. You’ll notice they have thicker bases and walls to handle increased stress from carbonation. Also, some highly carbonated brews will forgo the simple bottle cap and opt instead for a “cork and cage” stopper to maintain integrity.)
DIY Battery
Back in the old days before the superlab or the brilliant mobile meth-cooking scheme of Vamonos Pest, Walt and Jesse took the Winnebago out to the middle of the New Mexico desert and cooked up batches as needed. All in all a great plan until Jesse left the key in the ignition for a couple of days, which resulted in a dead battery and a stranded meth-lab on wheels just waiting to be found by the authorities. When their cellphone dies, their spare generator bursts into flame and their only hope of salvation gets lost in the maze of unmarked desert roads, Walt resorts to science to save their bacon by cobbling together a makeshift battery in order to jumpstart the Winnie. But would his scheme really have worked?
While Walt’s science is sound, he might have been better off attempting to juice up the cellphone, which would take way less electrochemical power than jumpstarting an RV. Let’s start with the theory. A battery is a self-contained power supply that both generates and stores electrical energy via chemical reactions. Composed of a series of fuel cells (or Galvanic cells, which convert free energy from a chemical reaction into electrical energy), a battery’s basic functional parts have the following components: anode, the negative electrode that holds charged ions; cathode, the positive electrode that holds discharged ions; an electrolytic (ie salt) solution that allows ions to move from the anode to the cathode; and a conductor to carry the electrons, usually a metal wire. What kind of metal makes the best wire?
Now that we’ve got the basics of the design down, here’s Walt’s piecemeal design: for the anode, he gathered galvanized metal composed mostly of zinc in the form of coins, nuts, bolts and washers; the cathode was made from the Winnebago’s brake pads which were composed of mercuric oxide and graphite; the electrolyte was a sponge soaked in potassium hydroxide, with the above-seen copper wire as a conductor. (Random thought: since Jesse introduced the story of a man lost in the wild who survived by drinking his own urine, I fully expected Walt to suggest using urine as the electrolyte since it’s chock full of aqueous salts and there’s a ready supply available. Perhaps that was a bit much for the censors…) Even though Walt made six of these fuel cells and attacked them to the Winnebago’s battery with jumper cables, would it have been enough to spark it up? Probably not. According to ScriptPhD.com, the makeshift battery would only have churned out about 12 volts and 20-30 amps of current at the most. The Winnie would have needed about 20 times that to get cranking.
Pardon Me, Waiter. I Appear to Have Some Ricin My Tea.
We already talked about the source of ricin earlier in this post, but since it was written before the finale, now we get to discuss whether or not it would have worked in the way Walt used it. It was a bit of an unexpected twist with Walt using the ricin to take out the annoying/eccentric/hit-happy Lydia Rodarte-Quayle (Laura Fraser). Lydia’s penchant for Stevia and soy milk in her Chamomile tea was well established, having been seen in a number of episodes leading up to the finale. Walt’s sleight of hand swapped Lydia’s Stevia for ricin, and she was none the wiser until it was far too late. But would this have worked?
Probably not. I mentioned above that ricin, when inhaled, needs only 1/228th of an aspirin tablet to prove deadly, while about 4 aspirin tablets worth would need to be orally ingested to achieve the same effect. Walt likely gave her that dose, probably equivalent to a Stevia packet and very similar in appearance to the sugar substitute. While Lydia could have been cured if she’d gone straight to the hospital, it’s more likely that the toxin wouldn’t have taken effect in the first place ... but why not?
The culprit in ricin is a lectin, as mentioned previously, which is a protein that interferes with cellular metabolism (ie an energy-producing and repair-inducing process). The issue with proteins is that their structure is extremely important to their function, so any change in the former will cause a change in the latter. Things that change protein structure include, and are not limited to, pH extremes, strong agitation, radiation and, most importantly, heat. To make a nod back to our homebrewing discussion, the addition of heat to the wort is highly controlled in order to make sure certain proteins survive long enough to do their job while others are denatured (ie unraveled) and inactivated. It’s likely that the near-boiling temperature of the water in Lydia’s Chamomile tea would have denatured the ricin lectins to the point of reduced or even total inactivity. Sorry, Walt!
FeLiNa
With one episode left in the epic run of Breaking Bad, fans were trying to figure out how it's all going to end. Attention turned to the final episode title, "Felina". Some thought it refered to the woman in Marty Robbins' song, "El Paso". Others thought it was simply an anagram of "finale". Still more want to pull the word apart by its elements: Fe - Li - Na. While Bromine Barium Breaking Bad has used the fun effect of highlighting letters in titles and names by blocking them off as their respective periodic table counterparts (the "V" in Vince Gilligan is for the element Vanadium), this theory might be a bit of a stretch. [They dropped a Ch in there for MiChael Slovis, which doesn't exist in the periodic table, but it could be a carbon-hydrogen bond.]
reddit, the rabbit hole of all internet rabbit holes, had theorized that FeLiNa can be broken down into its elemental representations: Iron, Lithium and Sodium. The theory went further, linking each element to Blood, Meth and Tears. Iron exits in hemoglobin, the oxygen-transporting molecule in red blood cells; lithium is a reducing agent in some processes of methamphetamine production, which has led some amateur cooks to grab it in abundance from batteries; sodium is a component in tears as the salt, sodium chloride; sodium is also a component in sweat, but Blood, Meth and Sweat doesn't sound quite so poetic. (Fun fact: Butterflies drink turtle tears to obtain sodium.)
Even if "Felina" wasn't as hard-hitting on the science front as the rest of the award-winning show was, it remains one of the greatest final hours for one of the best shows ever on TV. And if you learned a little science along with the watching, then all the better!